
IgA Nephropathy
History of IgAN
Contents
- Jean Berger – the man who identified IgA Nephropathy- John Feehally
- Berger’s disease before Berger – J Stewart Cameron
To discover more, go to:
Feehally J, Cameron JS IgA nephropathy: progress before and since Berger. Am J Kidney Dis. 2011 Aug;58(2):310-9
Jean Berger – the man who identified IgA Nephropathy (By John Feehally)
Although IgA nephropathy is the name most commonly used for the renal disease we study, many other terms have been used for it over the last 30 years. Most have derived from a description of the features of the disease found on renal biopsy: for example mesangial IgA disease, IgA glomerulonephritis, and IgA-IgG nephropathy.
But one title often used in the past for IgA nephropathy stands out: Berger’s Disease, named after Jean Berger, the French pathologist who published the first description of IgA nephropathy as we recognise it today.
At the time Jean Berger made and reported his seminal observations he was working as a pathologist in Paris at Hôpital Necker, and was also Professor at the Université René Descartes. In the late 1960’s renal biopsy was an increasingly used technique for investigating renal disease and the nephrologists with whom Berger worked at Hôpital Necker and Hôpital Tenon in Paris were among the leaders in this new wave of work. A classification of glomerulonephritis had been developed based on the various pathological appearances seen on specimens taken by renal biopsy, but in the mid-1960’s it was largely based on the morphology as seen on light microscopy. The new technique of immunofluorescence microscopy, undertaken with fresh renal biopsy material to identify the presence of immunoglobulins and complement components, was still regarded as an experimental technique. Berger applied the technique to renal biopsies and recognised that there was a group of patients, not properly defined before, in whom the dominant finding was the deposition of IgA in the glomerular mesangium. Electron microscopy showed there were mesangial electron dense deposits corresponding to the mesangial IgA. Light microscopy showed mesangial hypercellularity, which was usually focal and segmental. Interpreting his findings in the light of clinical information, he realised that typically these were young adults with low grade proteinuria and microscopic haematuria, and most often with recurrent episodes of macroscopic haematuria coinciding with upper respiratory tract infection.
The first public appearance of his findings was modest: an oral report to the French Société de Néphrologie in the winter of 1968, followed by the publication in French of a summary less than one page long (1). This report, now cited time and again all over the world, was entitled “Les dépôts intercapillaires d’IgA-IgG”. His co-author was an electron microscopist, Dr Nicole Hinglais.
At first the importance of Berger’s findings was poorly appreciated in many circles. A number of influential authorities doubted whether IgA nephropathy should be accorded the right to be identified as a distinct pattern of glomerulonephritis. Many of these patients, before Berger’s description of the immunofluorescence findings of IgA, would have been classified as having ‘focal nephritis’, a term in wide use at that time which some were reluctant to discard. [For a description of the development of the term focal nephritis, please read the adjacent article by Professor Stewart Cameron]. Rapidly other renal pathologists identified similar cohorts of patients, and it became clear that IgA deposition was indeed the commonest finding in patients with diffuse and focal mesangial proliferative glomerulonephritis; IgA nephropathy had been defined.
Berger followed his original descriptive phrase, dépôts intercapillaires d’IgA-IgG (1), by using the term ‘nephropathy with mesangial IgA-IgG deposits’ (2). But this was soon replaced by a number of other terms, including IgA nephropathy, mesangial IgA disease, mesangial IgA glomerulonephritis, IgA-IgG nephropathy of which IgA nephropathy has proved the most enduring. In the early years the condition was often called Berger’s disease, although this term was sometimes restricted particularly to those patients who had recurrent episodes of macroscopic haematuria.
Within a short time, Berger made further seminal observations. Firstly, he identified that similar mesangial IgA deposits also typified the glomerulonephritis associated with Henoch-Schönlein purpura (2), which indeed was morphologically often indistinguishable from IgA nephropathy. Secondly, he showed that recurrence of mesangial IgA deposits occurred frequently in kidneys transplanted into patients who had developed end-stage renal disease due to IgA nephropathy (3). Thirdly, Berger also published a major description of the secondary form of IgA nephropathy associated with alcoholic liver disease (4).
With these observations, Berger established a new understanding of these common groups of patients with glomerulonephritis, and it soon became clear that IgA nephropathy was the commonest pattern of glomerulonephritis found wherever renal biopsy was widely practiced. The glomerular disease in these patients could now be identified and classified, and studies of their pathogenesis, clinical course and treatment could begin to be established.
Since Berger’s observations were so innovative and influential, why has the term ‘Berger’s disease’ gradually fallen out of use? Chiefly perhaps because eponymous titles for diseases are becoming less fashionable. But we should not let future generations of nephrologists forget the seminal contribution made by Berger, even if his name no longer identifies the entity he discovered
Berger’s Disease Before Berger By J Stewart Cameron
When in 1968 Jean Berger and his colleagues in Paris described glomerular mesangial deposition of IgA in some nephritides, they did more than make a novel observation : they introduced a whole new level of descriptive classification into the study of clinical glomerulonephritis. This action illustrated only too well the morass of descriptions and classification of glomerular diseases had fallen into, which still awaits a full resolution. To understand what “Berger’s disease” might mean and how it evolved, we have to look back a long way.
Classifications of nephritis 1820-1970
Since Richard Bright’s universally-known book of 1827, and Gottlieb Gluge’s and Gabriel Valentin’s much less well-known pioneering studies of renal histology ten years later, the clinical manifestations and the macroscopic and microscopic appearances of nephritic kidneys (“Bright’s disease”) had been discussed by many observers. Bright’s work was done just before the new improved apochromatic microscopes were introduced into clinical practice, following which microscopy of renal tissue – and urine – became a regular feature of clinical enquiry (although Bright made one microscopical study in 1839-42 with Joseph Toynbee). The major advance of Valentin in 1837 was the invention of a double- bladed knife, which allowed the cutting of “thin” sections of tissue for examination (unstained) under the microscope.
Thus, ideas about nephritis were dominated from 1840 onwards by the combination of clinical observation and the macroscopic and microscopic observation of renal tissue obtained after the death of the patient. To begin with, tissues were unstained for microscopy; but the introduction of natural dyes (such as carmine) in the 1850s, and then synthetic stains from 1860 onwards meant that renal morphology dominated thinking. There had been an uneasy tension between this level of description and clinical observation from the start – very early it was noted that in one clinical setting (say dropsy and proteinuria) a number of different appearances could be seen in the kidney. These appearances were initially described in the 1840s and 1850s as either a fatty kidney with normal or nearly normal glomeruli, or in contrast a form in which the presence of cells and tubular destruction predominated. Debate about the relationship between these two forms, and their relationship to scarred granular kidneys, dominated renal pathology up to and beyond the First World War.
Pathologists naturally felt that their level of description was more fundamental than clinical classifications, and that the underlying “diseases” were expressed primarily in morphological terms. Thinking was – and remained – confused. Pathological terms (such as the notorious “nephrosis”) slipped sideways and came to be used for clinical syndromes, and vice versa. A further, apparently even more fundamental, layer of description was introduced when the aetiology of some of the appearances was teased out: “post- streptococcal nephritis” was the first, in the 1880s as bacteriology was born and the streptococcus identified ( “scarlatinal nephritis” had existed since 1740 or earlier).
Then the idea of time came to influence classification: first with the crude description of “acute” and “chronic” forms. Then came the “Dauerstadium” of nephritis, described by Volhard and Fahr, as did the “chronic latent nephritis” of Addis. As ideas of immunology developed, beginning with Schick’s landmark observations on streptococci, and then the work of von Pirquet on serum sickness in 1900-1910, immunological terms came into use: thus in the 1960s ” (chronic) hypocomplementemic nephritis” made its appearance and by analogy from studies of experimental nephritis in animals, different patterns of deposition of immune reactants such as complement and immunoglobulin [Ig] were seen: the “lumpy bumpy” and “linear” deposits which Germuth and Dixon correlated to different routes of immunopathogenesis. Finally from the 1950s onwards terms based on the results of the new effective treatments treatment such as “corticosteroid-sensitive” and corticosteroid-resistant” nephritis appeared. About this time also the advent of renal biopsy showed the plasticity of renal histological appearances.
The result of all this was great confusion for pathologists, doctors – and not the least – patients. I have described the process above as though it was a conscious process of layering. However absolutely the reverse was true, and in 1968 when Jean Berger and his colleagues differentiated aggregates visible on fluorescent microscopy by immunoglobulin class, IgA as opposed to IgG, chaos reigned.
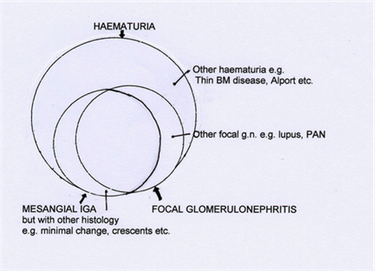
The clinic: recurrent or persistent haematuria, macroscopic or microscopic
Not surprisingly, bloody urine was know to the ancients, and is mentioned several times in the Hippocratic corpus. Its associations with stones and obstruction was even then well known. Less well known was that it could originate in the kidney itself, but in the seventeenth and especially the eighteenth century descriptions of acute post-infectious (usually post-scarlatinal) dropsy, oliguria and haematuria appeared, from Storch in 1742 to William Wells in 1808. Only when improved microscopes with apomorphic lenses allowed microscopy of urine and tissue to begin in the 1830s did Pierre Rayer in Paris, influenced by Donné’s classes in microscopy, observe urine with Eugène Vigla his student, and described apparently clear urine which persistently contained an excess of red cells. Probably the first decisive observation of bleeding from within the kidney itself was that of William Bowman in his classic paper of 1842, which included pathological as well as normal kidneys, and which demonstrated red cells within tubules – an observation repeated by Henle almost at once, who in addition correctly divined the origin of urinary casts.
During the latter half of the nineteenth century, urine microscopy produced data as good as any today, including observations of acanthocytes in renal bleeding as early as the 1880s. A familial incidence of persisting haematuria both microscopic and macroscopic, was noted also about the same time, and led later to recognition of Alport’s syndrome in the 1920s. Like all clinical renal syndromes, renal haematuria was obviously heterogeneous. About the same time patients with recurrent attacks of macroscopic haematuria were described, who had microscopic haematuria between the attacks. Probably the first such observations were those of E Wagner in 1882 and Nestor Tirard in London in 1899. However the study of glomerulonephritis was dominated by the presence and quantity of proteinuria – and the two had been know to co-exist in the urine at least since Wells in 1808. The presence or absence of haematuria did not form a major point in debates until after the First World War, and was not emphasised by Volhard and Fahr. This was to change in the 1920s when Thomas Addis described quantitative excretion of formed elements in the urine, including red cells, as a measure of the activity and/or progression of renal diseases. The “Addis count” as a surrogate for renal appearances during life was widely performed until renal biopsy became available 30 years later. Since recurrent macroscopic haematuria was more common in childhood, a substantial literature appeared in paediatric journals on this topic from 1950 to 1970.
Histology: Focal segmental glomerulonephritis
Volhard and Farr mentioned a case which may have been focal and segmental glomerulonephritis, and they quote a description of Sciedemandl in 1916, but the first papers to draw wide attention to this appearance were those of Baehr in the United States in the early 1920s. At first, focal nephritis was noted in patients with endocarditis, and thus described as “embolic”, a term which although quite erroneous persisted for decades. Then in 1926 Baehr described 14 young adults without endocarditis but with recurrent macroscopic haematuria, many of whom did well, some of whom had focal segmental nephritis. The idea of a focal nephritis remained contentious however, and was attacked amongst others by Payne and Illingworth working in children with acute forms of nephritis as late as 1940. In the papers by Ellis and Wilson from the London hospital in the 1940s, patients were described who would today be diagnosed clinically as IgA nephropathy. Despite which Wilson opposed the idea of focal nephritis when it re-surfaced in renal biopsy specimens a decade later.
It was not until the advent of renal biopsy in the 1950s that focal nephritis really came to attention. In 1957 Bates, Jennings and Earle as part of a study of acute nephritis, noted immediate post-pharyngitic macroscopic haematuria with proteinuria in 10 young soldiers with normal serum complement concentrations and normal ASOTs. Biopsies showed red cells in the tubules and generally mild, often focal segmental nephritis. However it was the papers two years later of Heptinstall and Joekes in London which focussed attention indelibly on this group of patients, demonstrating the variety of appearances associated clinically with a focal and segmental nephritis; only 3 of their 31 patients in 1960 showed recurrent haematuria as a presentation, many having Henoch-Schönlein purpura or lupus nephritis. Less attention has been paid to observations made by John Ross at the London hospital in 1960, focussing only on patients with long-term relapsing haematuria, and clearly defining this group of patients as an entity for the first time. Two papers appeared also describing similar cases in childhood: those of Bodian and Payne in 1962, and Ayoub and Bob Vernier in 1965. Thus by the 1960s focal, segmental glomerulonephritis was a recognised sub-group of proliferative glomerulonephritis. Finally during the late 1950s electron microscopy was first applied to renal biopsy material, and in 1962 Galle and Jean Berger noted “intercapillary” electron-dense material, presumed by analogy from animal work to be deposits of circulating immune complexes, in biopsies showing predominantly mesangial nephritis.
Immunohistochemistry: IgA deposition within the glomeruli
The idea of using fluorescent-labelled specific antibodies to detect and trace proteins appeared in the early 1950s, and by 1956 had been applied by Mellors and Ortega among others to post-mortem kidney specimens. In 1960 Freedman in Robert Kark’s laboratory had attempted successfully to use the same technology on the tiny fragments obtained by renal biopsy. Until the mid 1960s, however very few laboratories could undertake this technique, and the antisera used were often of poor specificity. By 1963 antibodies more or less directed against class-specific epitopes of the Ig light chains were available commercially, so that IgG IgA and IgM could be identified separately. However the few laboratories studying immunofluorescence in biopsies at that time mostly used only anti-IgG reagents, as this was thought to be the predominant immunoglobulin class involved in the immunopathogenesis of nephritis. For example, in the now little-known paper of Bodian from Great Ormond Street Hospital in 1965, biopsies from children with recurrent haematuria were studied using immunofluorescent techniques for the first time – but only antisera against whole Ig and IgG were used, and so an opportunity was surely missed. Then in 1968 two brief papers appeared from Jean Berger and Nicole Hinglais in Paris, describing predominant IgA mesangial deposition in some renal biopsies, although they emphasised that it was usually associated with some IgG. Then and now, there is of course no real comparative quantification of the different antisera to allow accurate comparison of the intensity of immunofluorescence, but in these patients the IgA reagent “outshone” the IgG reagent strongly. IgA nephropathy was born as a concept. Berger had trained with pathologist Deborah Doniach in London, where he got the idea of applying fluorescent techniques to kidney disease as she had to liver problems. At that time, Berger was working at the Necker Hospital in Paris. It should be noted also that he and his colleagues had the advantage of a pure anti-IgA antibody prepared by immunologist Maxime Seligmann, who had described anti-DNA antibodies for the first time a decade previously.
The three come together
As the introduction implied, when this new concept appeared in 1968 the confusion in ideas on the classification and pathogenesis of nephritis became even worse. After Berger and his colleagues published two short notes in French on the subject in 1968, there appeared a longer paper with illustrations, which rather unusually for such a subject was published in Transplantation Proceedings, then a new journal in its first year. In this paper 55 patients with various forms of glomerular morphology were described, most focal glomerulonephritis. All 55 had only minor proteinuria, but every one had microscopic haematuria, of whom 22 had one or more ’bouts of gross haematuria’. IgA was found also in Henoch-Schönlein purpura, a result already reported in the same year in the United States by Urizar, working with Al Michael and Bob Vernier. IgA was found by Berger also in patients with lupus nephritis, an observation made previously in the US by Koffler and others in 1969.
The world of nephrology remained a little sceptical for a while, however. Other papers from Paris emerged from Philippe Druet, and Liliane Morel-Maroger (who had worked with Berger from 1966) together with Gabriel Richet, so that for a short while the disease seemed to be confined to France, and in 1973, Renée Habib, Micheline Lévy and their colleagues used in print for the first time (so far as I can ascertain) the term “Berger’s disease”. As John Feehally remarks in the companion article, Jean Berger has always remained rather embarrassed about this, as we know being modest and retiring to the extreme.
Then after a gap, in 1972-3 several papers from outside France appeared (from Maintz and colleagues in the Netherlands, Clark West and Peter Burkholder in the USA, and David Davies in London amongst others), then from Ueda in Japan and Australia from Andrew Woodroffe, and very quickly the finding of predominant mesangial IgA in haematuric patients with mesangial or focal nephritis was realised to be common world-wide. Nevertheless, in all only a dozen or so papers were published on the subject up to 1975, and only a few dozen during the late 1970s: but after this interest expanded rapidly, and more than 600 appeared from 1981 to 1988. In part, this may have been the result of immunofluorescence techniques being far from universally available in renal histology laboratories even during the early 1970s, workers needing to fluorosceinate their own antibodies before use.
However by 1975 the salient features of IgA nephropathy were established: a condition with moderate proliferative glomerular changes, usually mesangial but often focal or segmental, associated with haematuria often macroscopic and red cell casts, and by 1974 were known to be associated with an elevated IgA concentration in the plasma. Evolution was stable or slow, but renal failure, increasing proteinuria and hypertension did occur eventually in some patients. When such patients were transplanted, Berger showed in 1975 that IgA rapidly recurred in the allografted kidney in about half the recipients, but that not all grafts failed because of this.
The role of the IgA, or lack of it, in the evolution of the associated nephritis has been the subject of 30 years’ more debate, which continues today. This and many other topics are the subjects of study by members of the International IgA Nephropathy Network.
References
- Addis TA. Glomerular nephritis. McMillan, New York, 1948
- Ayoub EM, Vernier RL. Benign recurrent hematuria. Am J Dis Child 1965 109: 217-223.
- Baehr G. A benign and curable form of acute hemorrhagic nephritis. JAMA 1926; 86: 1001- 1004.
- Bates RC, Jennings RB, Earle DP. Acute nephritis unrelated to groupA hemolytic streptococcus infection. Am J Med 1957; 23: 510-528.
- Berger J. IgA glomerular deposits in renal disease. Transpl Proc 1969; 1: 939-944
- Berger J, Yaneva H, Nabarra B, Barbanel C. reurrence of mesangial deposition of IgA after renal transplantation. Kidney Int 1975; 7:232-241.
- Berger J, Hinglais N. Les dépôts intercapillaires d’IgA-IgG. J Urol Nephrol 1968; 74: 694- 695.
- Bodian M, Black JA, Kobayashi N, Lake BD, Shuler SE. Recurrent haematuria in childhood. Q J Med 1965; 34: 359-382.
- Bowman W. On the structure and use of the Malpighian bodies of the human kidney with observations on the circulation through that gland. Phil Trans Roy Soc London 1842; 132: 57-80.
- Bright R. Reports of medical cases Vol I. Longman, Orme Rees London 1827.
- Davies DR, Tighe JR. Recurrent haematuria and glomerular IgA deposition. J Clin Pathol 1973; 26: 672-677
- Druet Ph, Bariéty J, Bernard D, Lagrue G. Les glomerulonéphrites primitives à dépôts mésangiaux d’IgA et d’IgG. Étude clinique et morphologique Presse Méd 1970; 78: 583-587.
- Ellis AW. Natural history of Bright’s disease. Clinical, histological and experimental observations. Lancet 1941; i : 1-7
- Freedman P, Peters JH, Kark RM. Localization of gamma globulin in the diseased kidney. Arch Intern Med 1960; 105:524-535.
- Galle P, Berger J. Dépôts fibrinoïdes intercapillaires. J Urol Néphrol 1962; 68: 123-127.
- Gluge G. Anatomische-mikroskopische Untersuchungen zur allegmeinem Anatomie und Pathologie. Jena 1842. Pp 126-131
- Heptinstall RH, Joekes AM. Focal glomerulonephritis. A study based on renal biopsies. Q J Med 1959 28; 329-346.
- Hyman LR, Wagnild JP, Beirne GJ, Burkholder PM. Immunoglobulin-A distribution in glomerular disease: analysis of immunofluorescence localization and pathogenetic significance. Kidney Int 1973; 3: 397-408.
- Koffler D, Agnello V, Carr RI, Kunkel HG. Variable pattern of immunoglobulin and complement deposition in the kidney of patients sith systemic lupus erthematosus. Am J Pahol 1969; 56: 305-316
- Levy M, Beaufils H, Gubler MC, Habib R. Idiopathic recurrent macroscopic haematuria and mesangial IgA-IgG deposits in children (Berger’s disease). Clin Nephrol 1973; 1: 63-69.
- McEnery PT, McAdams J, West CD. Glomerular morphology, natural history and treatment of children with IgA-IgG mesangial nephropathy. In Mathew T, Kincaid-Smith P (eds) Glomerulonephritis. Morphology, natural history and treatment. John Wiley, New York 3 vols, pp 305-320.
- Maintz, J, Elema JD, Henningsen B et al. Ein Sonderform der chronischen Glomerulonephritis IgA-IgG nephropathie. Deutch Med Wschr 1972; 97: 1527-1533.
- Mellors RCL, Ortega LG, Holman HR. Role of gamma globulins in the renal lesions of systemic lupus erythematosus and chronic membranous glomerulonephritis, with an observation on the lupus erythematosus cell reaction. J Exp Med 1957; 106: 191-201.
- Morel-Maroger L, Leathem A, Richet G. Glomerular abnormalities in non-systemic disease. Am J Med 1972; 53; 170-184.
- Payne WW, Illingworth RS Acute nephritis in childhood with special reference to the diagnosis of focal nephritis. Q J Med 1940; 9: 37-42.
- Ross JH. Recurrent focal nephritis. Q J Med 1960; 29: 391 –406.
- Storch J. Prakticher und theoretiker Traktat vom Scharlachfieber. C Mevius, Gotha, 1742 pp 238-240. Daniel Sennert (Sennert D. De febribus libri IV: Acessit ad calcem, ejusdem de dysenteria tractatus. Ed Novisssiumua, cui accessit fasciculus medicamentum contra pestorum. F Baba Venice, 1641 p. 178) had described dropsy after scarlatinia a century earlier, but seems to have missed the haematuria. The description of the urine “as though meat had been washed in it” quoted for a century or more seems to originate with N Rosen von Rosenstein in 1765 (Underrattelser om bajrnsjukdomar och deras botmedel. Stockholm).
- Tirard N. Albuminuria and Bright’s disease. Smith Elder & Co , London 1899. P. 152
- Toynbee J. On the intimate structure of the human kidney and on the changes of its several component parts undergo in Bright’s disease. Med Chir Trans 1846; 29: 304-326.
- Ueda Y, Sakai O, Yamagata M, Kitajima T, Kawamara K. IgA glomerulonepritis in Japan. Contrib Nephrol 1973; 4: 37-47.
- Urizar RE. Michael A. Sisson S, Vernier RL. Anyphylactoid purpura II. Immunofluorescent and electron microscopic study of the glomerular lesions. Lab Invest 1968; 19: 437- 450
- Valentin G. Repertorium für Anatomie und Pathologie. Huber, Bern St Gall 1837; 11(2): 290-291.
- Vigla EN. Nouvelles observations sur l’étude microscopique de l’urine, eclairée par l’analyse chimique. L’Expérience 1837; 12: 177-190; ibid 1838; 13: 193-204; ibid 1838; 26: 401-412, 27: 417-425
- Volhard F, Fahr T. Die Brightsche Nierenkrankheit. Springer Berlin 1914
- Wagner EV. Handbuch der Krankheiten der Harnapparatus. CW Vogel, Leipzig, 1882.
- Wells WC. Observations on the dropsy, which succeeds scarlet fever. Trans Soc Improve Med Chir Knowl 1812; 3: 167-186. (given November 4th 1806)
- Woodroffe AJ, Thomson NM, Meadows R, Lawrence JR. IgA-associated glomerulonephritis. Aust NZ J Med 1975; 5: 97-100
